Digital Health Applications (DiGA)
Scientific services for digital health application manufacturers, according to §33a and §139e SGB V
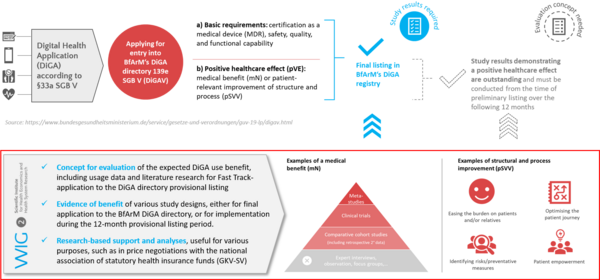
Background: "Fast Track" into the DiGA directory
The Digital Health Care Act (DVG) came into force in Germany on December 19th, 2019 and reinforces the role of so-called digital health applications (DiGA according to §33a and §139e SGB V) as an increasingly important aspect of our healthcare system.At the Federal Institute for Drugs and Medical Devices (BfArM), a new application pathway for inclusion in standard care was initiated for these innovations from May 2020: the "fast track" to the DiGA directory according to §139e SGB V. Innovative digital health services can thus be included for the first time in the catalogue of services reimbursable by the statutory health insurance (SHI) - if certain criteria are met. In particular, proof of safety, quality, function (certification according to MDR in risk class I or IIa) and positive care effects (pVE) is necessary for such inclusion. These requirements are regulated by the Digital Health Applications Ordinance (DiGAV). The BfArM's decision on inclusion should be available via the online portal no later than three months after application. A special feature: If a study on the pVE is not yet available, a temporary inclusion in the standard care for testing the application over a period of 12 months is also possible. During this period, the evaluation of the proof of benefit must be carried out by an independent institution on the basis of an evaluation concept submitted with the application.For the manufacturers and providers of digital health services, this opens up a new, promising opportunity to enter the German health care market independently of the often small-scale selective-contractual or the sphere of privately financed health care services.
We have compiled a detailed list of all relevant aspects concerning the positive care effect (pVE) according to DVG/ DiGAV in our free DiGA checklist.
We provide scientific and independent evaluations. Using appropriate research-oriented analyses and consulting services, we support digital health application (DiGA) manufacturers on their way into the reimbursable standard care (SHI) in Germany via the fast-track application process.
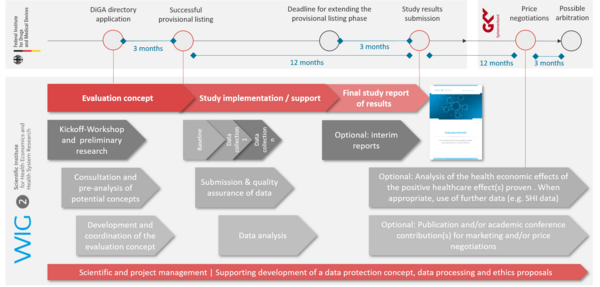
Creating evaluation concepts
Digital health application (DiGA) manufacturers can apply for provisional listing in the DIGA directory, even when final study results proving a positive care effect cannot yet be presented, if provided that the effect is plausible. While listed provisionally, evidence of a positive care effect must be supplied, and two requirements must be met:
1. Expected positive care effect ("pilot study")
Based on a systematic evaluation of usage data of the digital application, initial indications for the study to be conducted are to be derived. In parallel, the BfArM requires an initial literature search on comparable study subjects. The key points identified in this way should contain statements on the expected intervention effects, case numbers, measurement instruments, recruitment methods and relevant questions or study endpoints.
We support DiGA providers in planning and analysing the systematic user data evaluation and ensure that results meet the BfArM formal requirements; fulfilling evaluation criteria for the fast-track application.
2. Submitting a scientific evaluation concept
The application for provisional admission must be accompanied by a binding evaluation concept, created in accordance with international scientific standards by an independent institution. The evaluation has to be feasible within the 12-month trial period. The concept must be suitable for demonstrating the defined patient benefit (positive care effect) and must address the results of the preliminary analysis (pilot study).
We create an individual evaluation concept for application to the DiGA directory. The concept will meet all the BfArM manufacturer guideline criteria and entails thorough preliminary research, coordination, and review with the client (including a kick-off workshop). Information on study design, methods, case number planning and recruitment as well as dates for data collection, analyses and the results report will all be part of the concept. We also provide support prior to application submission, taking part in a recommended consultation appointment with BfArM.
Implementing the evaluation study
The positive care effect (pVE) of a DiGA must be proved by a study, either as a medical benefit (mN) or a patient-relevant improvement of structures and processes (pSVV). This must be provided for a clearly defined patient group (by 3- or 4-digit ICD-10 code), and for at least one healthcare effect related to the intended purpose of the application. In the evaluation study, at least one comparative, retrospective analysis must be performed, in which the comparison group reflects the care reality (treatment without the DiGA, non-treatment, or treatment with a comparable DiGA). The study must be conducted in Germany, must be registered with the public and published as a study report that meets international standards. For further details on study requirements, please see our detailled DiGA checklist for proving a positive healthcare effect.
As an independent institution and evaluator, we support DiGA manufacturers with an compliant evaluation study. Building on the evaluation concept and study design, we run quality assurance and analyse study data at several data collection points, presenting final study results in a final study report for publication and submission to the BfArM. We as well provide project management throughout the entire study implementation and offering support with data protection and ethics proposals.
Additional health economic analyses
Evaluation of a DiGA is to be carried out exclusively on the basis of patient-relevant endpoints. The purpose is to prove a positive healthcare effect and thus be included in the reimbursable healthcare services DiGA directory (according to §139e in the fifth social insurance code book (SGB V)).
However, a benefit analysis from the perspective of other stakeholders is also recommended – particularly regarding costs. At the very least, an analysis of the DiGAs economic effects is relevant in price negotiations with the national association of statutory health insurance funds (GKV-Spitzenverband). The results obtained according to scientific standards also provide support in negotiations in the context of, or beyond, the DiGA Fast Track, such as contracts with individual health insurance companies (§68 and 140a SGB V).
We conduct health economic analyses in accordance with the medical device purpose, indication and the expected/proven care effects of the DiGA, with the goal of identifying positive cost effects. The focus is typically on statutory health insurance (SHI) system expenditure optimisation. The WIG2 Institute has profound experience and a suitable database for this purpose.
Publications or academic conference contributions
The application for inclusion in the DiGA directory in accordance with §139e SGB V calls for the publication of the study results as a scientific evaluation report (Clinical Study Report, CSR), demonstrating positive healthcare effect. The publication must occur completely online—for example, on the DiGA provider’s website.
It may, however, also be useful to further publish the study results; for subsequent price negotiations, initiating further collaborations, or for PR and sales purposes. Scientific publications, and academic conference papers and/or posters can be a convincing supplement and lead to increased awareness, acceptance and ultimately willingness of various stakeholders to invest.
WIG2 prepares scientific publications of study results, as well as of the entire submission and review process with an appropriate peer-reviewed journal. The article will be created and published with authors from the DiGA provider, WIG2 Institute scientific staff and, if deemed necessary, with the involvement of external experts. Alternatively, the results of the evaluation can be prepared and submitted through academic conference contributions and/or posters assembled by the WIG2 Institute.
References
Successfully listed in the DiGA-Directory
Further DiGA-Projects
2021 - DiGA evaluation concept for an symptom monitoring app
2021 - DiGA evaluation concept for an addication treatment app
2021 - DiGA evaluation concept for a rehabilitation app
2021 - DiGA evaluation concept for a COPD treatment app
2021 - DiGA evaluation concept for a VR application
2021 - DiGA evaluation concept for a cancer support app
Publications
Blaschka, M., Häckl, D., Schönfelder T. (2021). Das Erstattungsverfahren - Evaluation. In Jorzig, A. & Matusiewicz, D. [Hrsg.]: Digitale Gesundheitsanwendungen (DiGA) - Rechtliche Grundlagen, digitale Technologien und digitale Köpfe. Heidelberg: medhochzwei Verlag.
Blaschka, M., Häckl, D., Schönfelder T. (2020). Wege über die Forschung in die gesetzliche Erstattungsfähigkeit – Digitale Versorgungsinnovationen. In von Dercks, N. [Hrsg.] Operatives und strategisches Medizincontrolling. Kulmbach: Mediengruppe Oberfranken - Fachverlage.
Expert lectures and workshops (selected)
2019/09 - World Café: How to get your idea into standard care? (HEALTHCARE HACKATHON)
2020/01 - Discussion Panel: Digital Care Act (HEALTH INSURANCE HACK&CON 2020)
2020/05 - Webinar: DiGA and Q&A positive care effect (Healthcare Innovators Meetup)
2020/07 - Webinar series: DiGA360° (Initation and Organisation; ongoing Format)
2020/08 - Workshop: The long road into the first german health market (Vision Health Pioneers)
2020/08 - DiGA-Wargame: Health insurance funds as enabler (BVMed e.V. / BPI)
2020/09 - Workshop: Digital Care Act (MSD Gesundheitsforum)
2020/12 - Online conference: Quo vadis, MDR and DVG? (Stadt Leipzig)
2020/12 - Virtual Roundtable on innovative evidence generation (Digtital Medicine Society & health innovation hub)
2021/3 - Status Quo on DiGA in Germany (Saxony Economic Development Corporation)
2021/6 - Future of Digital Therapeutics, Focus: DiGA (D-Health Innovation Tour: D-A-CH & Israel)
Others
Blaschka, M. (2020). Für die Gesundheit: Apps auf Rezept (Interview). In: AOK PLUS-Blog. Online: https://presseblog.aokplus-online.de/fuer-die-gesundheit-erste-apps-auf-rezept-moeglich/
2021 - Blaschka, M. & Schönfelder, T. Ein Hoch auf den Goldstandard (Gastbeitrag): In: Tagesspiegel Background Gesundheit & eHealth. Online: https://background.tagesspiegel.de/gesundheit/ein-hoch-auf-den-goldstandard
Um diese Seite auf Deutsch zu sehen, klicken Sie hier.
Download: DiGA checklist pVE
Download our free DiGA checklist for proving a positive care effect (pVE) by clicking here (945 KB).

Dr. Tonio Schönfelder
Head of Healthcare Research
E-Mail: tonio.schoenfelder(at)wig2(dot)de
Tel.: + 49 341 3929 40-21